
The link between iron, erythrocytosis, and polycythemia vera outcomes
Erythrocytosis leads to iron dysregulation and increased TE risk in polycythemia vera (PV)
Since approximately 60% of patients show laboratory evidence of iron dysregulation as measured by low serum iron, TSAT, and ferritin, there is a critical unmet need to address iron dysregulation in PV.5,52
Understanding the iron–hepcidin connection7,38,52
Iron is absorbed from dietary sources, stored, and exported via a complex system involving duodenal, hepatic, and splenetic systems.52,60
Hepcidin, a polypeptide hormone produced in the liver, is the primary regulator of iron homeostasis, controlling iron absorption, distribution, and storage.38,52
Ferroportin is the sole iron exporter in mammals, transporting iron from enterocytes, splenic macrophages, and hepatocytes to the serum.52,61
The hepcidin–ferroportin axis is crucial for maintaining a balance between iron absorption, iron recycling, and iron utilization, particularly for erythropoiesis. Since the hallmark of PV is erythrocytosis, imbalance in this axis can further impact RBC production.6,38,52,62
Iron is essential for erythropoiesis, and PV is characterized by dysregulated iron metabolism
erythrocytosis despite iron deficiency caused by hepcidin suppression, since
under normal physiological conditions hepcidin would be downregulated to
recover from systemic iron deficiency.52
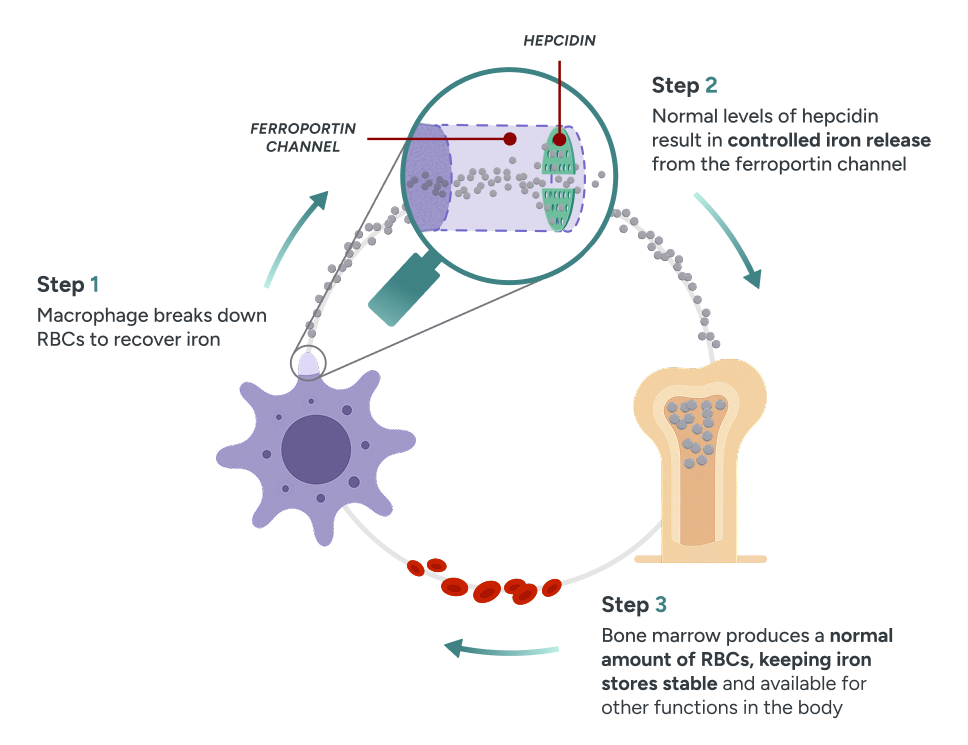
Normal physiology
Regulated iron metabolism
In normal homeostasis, binding of hepcidin to ferroportin blocks iron exportation, maintaining a feedback loop that effectively recycles unused iron from RBCs for erythropoiesis.52,62
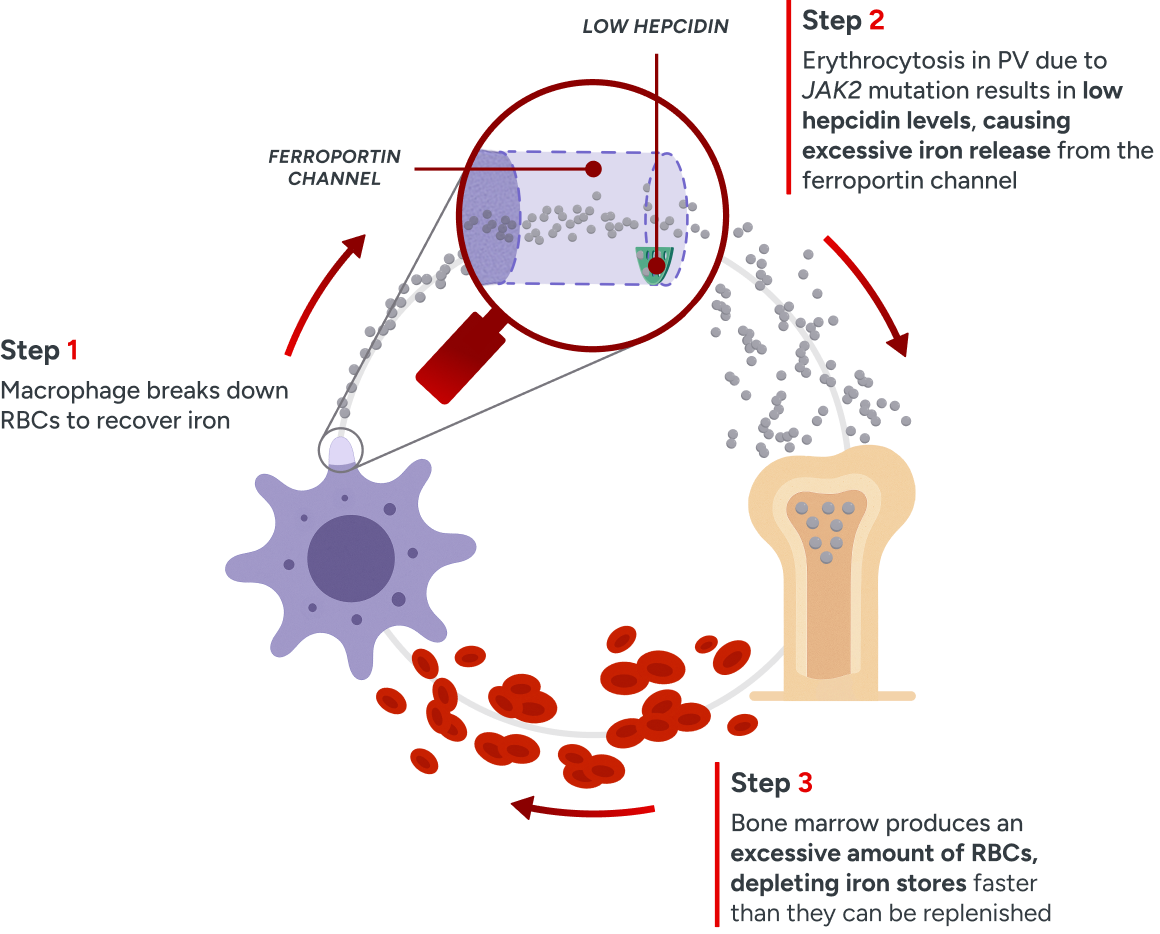
PV pathophysiology
Dysregulated iron metabolism
Erythrocytosis in PV leads to persistently low hepcidin levels, disrupting the iron feedback loop and thus increasing ferroportin activity. This leads to increased iron export from storage for erythrocytosis, ultimately contributing to systemic iron deficiency.52
Iron deficiency may persist or worsen with ongoing therapeutic phlebotomy
These symptoms are often driven or exacerbated by systemic iron deficiency, even in the absence of anemia.6,7
References
1. Verstovsek S, Pemmaraju N, Reaven NL, et al. Real-world treatments and thrombotic events in polycythemia vera patients in the USA. Ann Hematol. 2023;102(3):571-581. doi:10.1007/s00277-023-05089-6. 2. Verstovsek S, Pemmaraju N, Reaven NL, et al. Real-world treatments and thrombotic events in polycythemia vera patients in the USA. Electronic Supplemental Material. Ann Hematol. 2023;102(3):571-581. doi:10.1007/s00277-023-05089-6. 3. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500. 4. Poullet A, Busque L, Sirhan S, et al. Symptom burden in myeloproliferative neoplasms: clinical correlates, dynamics, and survival impact—a study of 784 patients from the Quebec MPN research group. Blood Cancer J. 2025;15(1):51. doi:10.1038/s41408-025-01234-8. 5. Randrianarisoa RMF, Ramanandafy H, Mania A, et al. Prevalence and diagnostic performance of iron deficiency in polycythemia. Hematology. 2023;28(1):2204621. doi:10.1080/16078454.2023.2204621. 6. Kuykendall AT, Fine JT, Kremyanskaya M. Contemporary challenges in polycythemia vera management from the perspective of patients and physicians. Clin Lymphoma Myeloma Leuk. 2024;24(8):512-522. doi:10.1016/j.clml.2024.04.003. 7. Ginzburg YZ, Feola M, Zimran E, et al. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105-2116. doi:10.1038/s41375-018-0207-9. 8. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667-679. doi:10.1182/blood-2016-10-695940. 9. Grunwald MR, Burke JM, Kuter DJ, et al. Symptom burden and blood counts in patients with polycythemia vera in the United States: an analysis from the REVEAL study. Clin Lymphoma Myeloma Leuk. 2019;19(9):579-584.e1. doi:10.1016/j.clml.2019.06.001. 10. Mehta J, Wang H, Iqbal SU, et al. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500. 11. Tefferi A, Barbui T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(9):1465-1487. doi:10.1002/ajh.27002. 12. Zhao ZJ, Vainchenker W, Krantz SB et al. Role of tyrosine kinases and phosphatases in polycythemia vera. Semin Hematol. 2005;42(4):221-229. doi:10.1053/j.seminhematol.2005.05.019. 13. Ling B, Xu Y, Qian S, et al. Regulation of hematopoietic stem cells differentiation, self-renewal, and quiescence through the mTOR signaling pathway. Front Cell Dev Biol. 2023;11:1186850. doi:10.3389/fcell.2023.1186850. 14. Waggoner M. Polycythemia Vera: Thinking Beyond the Hematocrit. J Adv Pract Oncol. 2023;14(5):405-413. doi:10.6004/jadpro.2023.14.5.5. 15. Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(5):1071-1082. doi:10.1007/s00277-019-03625-x. 16. MPN Research Foundation. Polycythemia Vera (PV). Updated 2025. Accessed August, 2025. Available at: https://www.mpnresearchfoundation.org/mpn-research/polycythemia-vera. 17. Leukemia & Lymphoma Society. Polycythemia Vera Facts. Revised April, 2015. Accessed August, 2025. Available at: https://www.lls.org. 18. Leukemia & Lymphoma Society. Myeloproliferative Neoplasms: In Detail. Revised 2025. Accessed August, 2025. Available at: https://www.lls.org. 19. National Organization for Rare Disorders (NORD). Polycythemia vera. Revised November, 2023. Accessed August, 2025. Available at: https://rarediseases.org/rare-diseases/polycythemia-vera/. 20. Cuthbert D, Stein BL. Polycythemia Vera-Associated Complications: Pathogenesis, Clinical Manifestations, And Effects On Outcomes. J Blood Med. 2019;10:359-371. doi:10.2147/JBM.S189922. 21. Brabrand M, Frederiksen H. Risks of Solid and Lymphoid Malignancies in Patients with Myeloproliferative Neoplasms: Clinical Implications. Cancers. 2020;12(10):3061. doi:10.3390/cancers12103061. 22. Landtblom AR, Bower H, Andersson TML, et al. Second malignancies in patients with myeloproliferative neoplasms: a population-based cohort study of 9,379 patients. Leukemia. 2018;32(10):2203-2210. doi:10.1038/s41375-018-0027-y. 23. Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients' overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167. doi:10.1186/s12885-016-2208-2. 24. Mesa RA, Miller CB, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with myeloproliferative neoplasms (MPNs) and hematologists/oncologists in the United States: findings from the MPN Landmark survey. Cancer. 2017;123(3):449-458. doi:10.1002/cncr.30325. 25. National Cancer Institute. Coping With Cancer: Emotions and Cancer. Updated April, 2025. Accessed August, 2025. Available at: https://www.cancer.gov/about-cancer/coping/feelings. 26. Bradford A, Young K, Whitechurch A, et al. Disabled, invisible and dismissed—The lived experience of fatigue in people with myeloproliferative neoplasms. Cancer Rep (Hoboken). 2023;6(1):e1655. doi:10.1002/cnr2.1655. 27. Verstovsek S, Harrison CN, Kiladjian JJ, et al. Markers of iron deficiency in patients with polycythemia vera receiving ruxolitinib or best available therapy. Leuk Res. 2017;56:52-59. doi:10.1016/j.leukres.2017.01.032. 28. Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199-1203. doi:10.1016/j.leukres.2009.01.035. 29. Cleveland Clinic. Polycythemia Vera. Revised April, 2022. Accessed August, 2025. Available at: https://my.clevelandclinic.org/health/diseases/9223-polycythemia-vera. 30. Barbui T. Appropriate management of Polycythemia Vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Hematol Transfus Cell Ther. 2022;44(1):S3-S4. doi:10.1016/j.htct.2022.09.1190. 31. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. N Engl J Med. 2004;350(2):114-124. doi:10.1056/NEJMoa035572. 32. Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia. 2021;35(12):3339-3351. doi:10.1038/s41375-021-01401-3. 33. Visweshwar N, Fletcher B, Jaglal M, et al. Impact of Phlebotomy on Quality of Life in Low-Risk Polycythemia Vera. J Clin Med. 2024;13(16):4952. doi:10.3390/jcm13164952. 34. Silver RT, Abu-Zeinah G. Polycythemia vera: aspects of its current diagnosis and initial treatment. Expert Rev Hematol. 2023;16(4):253-266. doi:10.1080/17474086.2023.2198698. 35. Kim KH, Oh KY. Clinical applications of therapeutic phlebotomy. J Blood Med. 2016;7:139-144. doi:10.2147/JBM.S108479. 36. Data on file. Takeda Pharmaceuticals U.S.A., Inc. 37. Edahiro Y, Komatsu N. Iron deficiency and phlebotomy in patients with polycythemia vera. Int J Hematol. 2025;121(1):39-44. doi:10.1007/s12185-024-03868-z. 38. Bennett C, Jackson VE, Pettikiriarachchi A, et al. Iron homeostasis governs erythroid phenotype in polycythemia vera. Blood. 2023;141(26):3199-3214. doi:10.1182/blood.2022016779. 39. Assi TB, Baz E. Current applications of therapeutic phlebotomy. Blood Transfus. 2014;12 (Suppl 1):s75-83. doi:10.2450/2013.0299-12. 40. Mayo Clinic. Polycythemia Vera. Updated May, 2025. Accessed August, 2025. Available at: https://www.mayoclinic.org/diseases-conditions/polycythemia-vera/diagnosis-treatment/drc-20355855. 41. Parasuraman S, DiBonaventura M, Reith K, et al. Patterns of hydroxyurea use and clinical outcomes among patients with polycythemia vera in real-world clinical practice: a chart review. Exp Hematol Oncol. 2016;5:3. doi:10.1186/s40164-016-0031-8. 42. Alvarez-Larrán A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363-1369. doi:10.1182/blood-2011-10-387787. 43. Jakafi (ruxolitinib). Package insert. Incyte Corporation; 2023. 44. BESREMi (ropeginterferon alfa-2b-njft). Package insert. PharmaEssentiaⓇ USA Corporation; 2024. 45. Vachhani P, Mascarenhas J, Bose P, et al. Interferons in the treatment of myeloproliferative neoplasms. Ther Adv Hematol. 2024;15:1-22. doi:10.1177/20406207241229588. 46. Tremblay D, Ronner L, Podoltsev N, et al. Ruxolitinib discontinuation in polycythemia vera: Patient characteristics, outcomes, and salvage strategies from a large multi-institutional database. Leuk Res. 2021;109:106629. doi:10.1016/j.leukres.2021.106629. 47. Chamseddine RS, Savenkov O, Rana S. Cytoreductive therapy in younger adults with polycythemia vera: a meta-analysis of safety and outcomes. Blood Adv. 2024;8(10):2520-2526. doi:10.1182/bloodadvances.2023012459. 48. Abelsson J, Andréasson B, Samuelsson J, et al. Patients with polycythemia vera have worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013;54(10):2226-2230. doi:10.3109/10428194.2013.766732. 49. American Cancer Society. Adjusting to Life with Cancer. Updated January, 2019. Accessed August, 2025. Available at: https://www.cancer.org/cancer/survivorship/coping/adjusting-to-life-with-cancer.html. 50. Ponce RKM, Verma K, Gergen-Barnett K, et al. A review of medical mistrust across the cancer continuum of care and current interventions. J Community Health. 2025;50(4):750-760. doi:10.1007/s10900-025-01462-w. 51. Poullet A, Busque L, Sirhan S, et al. Symptom burden in myeloproliferative neoplasms: clinical correlates, dynamics, and survival impact—a study of 784 patients from the Quebec MPN Research Group. Supplemental Figure 1. Blood Cancer J. 2025;15:51. doi:10.1038/s41408-025-01234-8. 52. Handa S, Ginzburg Y, Hoffman R, et al. Hepcidin mimetics in polycythemia vera: resolving the irony of iron deficiency and erythrocytosis. Curr Opin Hematol. 2023;30(2):45-52. doi:10.1097/MOH.0000000000000747. 53. McFarland DC, Shaffer KM, Polizzi H, et al. Associations of physical and psychologic symptom burden in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Psychosomatics. 2018;59(5):472-480. doi:10.1016/j.psym.2018.01.006. 54. Yu J, Parasuraman S, Paranagama D, et al. Impact of Myeloproliferative neoplasms on patients' employment status and work productivity in the United States: results from the living with MPNs survey. BMC Cancer. 2018;18:420. doi:10.1186/s12885-018-4322-9. 55. National Institutes of Health (NIH). Talking to Your Doctor. Updated December, 2016. Accessed August, 2025. Available at: https://www.nih.gov/institutes-nih/nih-office-director/office-communications-public-liaison/clear-communication/talking-your-doctor. 56. Harrison CN, Ross DM, Fogliatto LM, et al. Patient and physician perceptions regarding treatment expectations and symptomatology in polycythemia vera: Insights from the Landmark 2.0 global health survey. Hemasphere. 2025;9(3):e70106. doi:10.1002/hem3.70106. 57. Manz K, Heidel FH, Koschmieder S, et al. Comparison of recognition of symptom burden in MPN between patient- and physician-reported assessment—an intraindividual analysis by the German Study Group for MPN (GSG-MPN). Leukemia. 2025;39(4):864-875. doi:10.1038/s41375-025-02524-7. 58. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098-103. doi:10.1200/JCO.2012.42.3863. 59. Almeida LR, Faustino D, Gameiro R, et al. Masked polycythemia vera and iron deficiency in a fertile‑age woman. Cureus. 2023;15(1):e33545. doi:10.7759/cureus.33545. 60. Saad HKM, Abd Rahman AA, Ab Ghani AS, et al. Activation of STAT and SMAD Signaling Induces Hepcidin Re-Expression as a Therapeutic Target for β-Thalassemia Patients. Biomedicines. 2022;10(1):189. doi:10.3390/biomedicines10010189. 61. Pilo F, Angelucci E. Vamifeport: Monography of the First Oral Ferroportin Inhibitor. J Clin Med. 2024;13(18):5524. doi:10.3390/jcm13185524. 62. Ganz T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18(2):171-182. doi:10.1016/j.beha.2004.08.020. 63. Cleveland Clinic. Iron-Deficiency Anemia. Revised December, 2024. Accessed August, 2025. Available at: https://my.clevelandclinic.org/health/diseases/22824-iron-deficiency-anemia. 64. Faruqi A, Zubair M, Mukkamalla SKR. Iron-Binding Capacity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 2, 2024.